You are here
Content
Research
Neurology
The main research focus of the Department of Neurology is the control of the autoimmunity of the central nervous system and immunotherapeutic approaches for patients with brain tumors, such as glioblastoma.
In recent years, we have identified key events in the metabolism of the essential amino acid tryptophan as an endogenous mechanism for the inhibition of immune responses in the context of brain tumors, as well as a key receptor for immunosuppressive endogenous tryptophan metabolites. These findings have provided a new perspective on the role of tryptophan metabolism in cancer and new drug targets, such as aryl hydrocarbon receptor (AHR) inhibitors. In collaboration with Bayer, AHR inhibitors were identified and validated in preclinical models for their anti-tumor activity as monotherapy and in combination with immune checkpoint inhibitors (Friedrich et al., Nat Cancer 2021; Kober et al., J Immunother Cancer 2023). In 2023, two phase 1 trials for the treatment of cancer with the aim of boosting the body's own anti-tumor immunity were completed.
Other studies are focused on the discovery of novel target antigens for immunotherapy of a type of brain tumor, glioma. The immunogenicity of key glioma-specific mutations has been demonstrated in preclinical models. Based on the work demonstrating the immunogenicity of the IDH1R132H driver mutation (Bunse et al., J Clin Invest 2015; Schumacher et al., Nature 2014), the multicenter NOA-16 study was completed, demonstrating the safety and immunogenicity of an IDH1R132H-specific peptide vaccine in glioma patients. In the DKTK-initiated multicenter NOA-21 study (Bunse et al., Neurol Res Pract 2022), which was completed in 2023, we are now investigating whether the parallel administration of the checkpoint inhibitor avelumab increases the efficacy of the peptide vaccine and which intratumoral resistance mechanisms influence the efficacy of this immunotherapeutic approach. First indications have already been obtained by demonstrating the immunosuppressive effects of the oncometabolite 2-hydroxyglutarate in preclinical models and tumor tissue (Bunse et al., Nat Med 2018). The aim is to use such T cell receptors for treatment with patient-autologous T cell receptor-transgenic T cells by adoptive transfer.
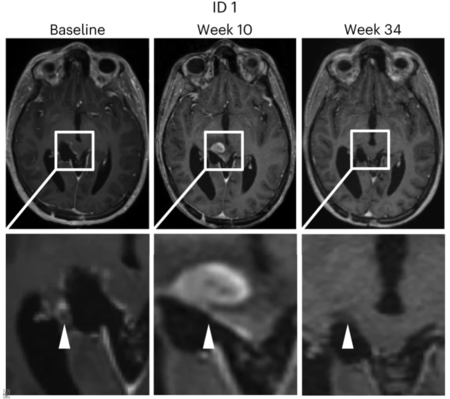
We are also investigating other glioma-specific neoepitopes such as H3K27M, in particular the identification of specific receptors on neoepitope-specific T cells activated in patients by vaccination. These T cell receptors will be specifically identified by single cell sequencing and functionally tested in a multi-center phase 1 trial INTERCEPT H3 (ClinicalTrials.gov identifier NCT04808245), funded by German Cancer Aid and conducted in collaboration with TRON (Mainz, Germany), in patients with H3K27M-mutated gliomas following vaccination against H3K27M in combination with the checkpoint inhibitor atezolizumab. The goal is to use such T cell receptors for the treatment of patients with autologous T cell receptor transgenic T cells by adoptive transfer. INTERCEPT H3 has already been successfully translated into the clinic and the first patients are being treated in this study (Grassl et al., Neurol Res Pract 2023). This experimental therapy has also already been scientifically evaluated in eight patients with diffuse midline gliomas (Grassl et al., Nat Med 2023), with the result that this vaccination proved to be safe and induced the desired immune responses directed against the brain tumor.
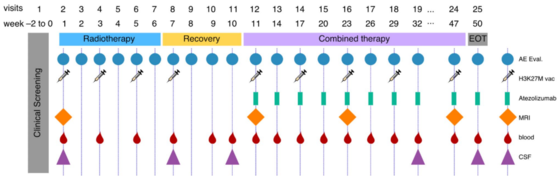
Intercept H3 treatment schedule: H3K27M-vac will be administered to 15 patients with newly diagnosed H3K27M mutant diffuse midline glioma concomitant to standard radiotherapy and subsequently in combination with atezolizumab. Longitudinal blood and cerebrospinal fluid (CSF) sampling will allow immunogenicity testing and three monthly MRIs together with regular clinical evaluation will be used to determine clinical response. Grassl et al., Neurol Res Pract 2023
Other collaborative research projects focus on the immunomodulatory effects of radiotherapy in brain tumors (CRC 1366), mechanisms of resistance to checkpoint inhibitors in gliomas (CRC 1389) and brain metastases (RTG 2099), the role of macrophages in gliomas (RTG 2727, SPP 2295) and the immune microenvironment of IDH-mutant gliomas (EU Glioresolve). In RTG 2727, we recently showed that natural killer cells, a cell type of the immune system, can impair the effect of cancer therapies with immune checkpoint inhibitors (Kilian et al., Sci Immunol 2024).
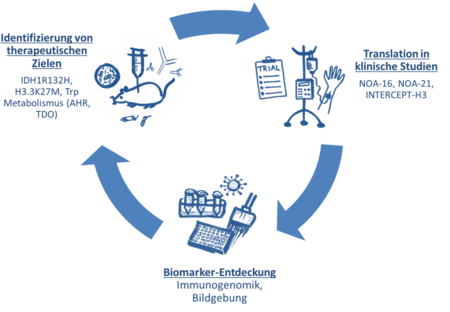
Iterative therapy development cycle. IDH1R132H - point mutation in the gene for isocitrate dehydrogenase type 1, H3.3K27M - point mutation in the histone-3 gene H3F3A, Trp - l-Tryptophan, AHR - aryl hydrocarbon receptor, TDO - tryptophan-2,3-dioxygenase.
Selected publications
*equal contribution
1. Kilian M, Friedrich MJ, Lu KH, Vonhören D, Jansky S, Michel J, Keib A, Stange S, Hackert N, Kehl N, Hahn M, Habel A, Jung S, Jähne K, Sahm F, Betge J, Cerwenka A, Westermann F, Dreger P, Raab MS, Meindl-Beinker NM, Ebert M, Bunse L, Müller-Tidow C, Schmitt M, Platten M. The immunoglobulin superfamily ligand B7H6 subjects T cell responses to NK cell surveillance. Sci Immunol. 2024. 9(95):eadj7970.
DOI: 10.1126/sciimmunol.adj7970
2. Grassl N, Poschke I, Lindner K, Bunse L, Mildenberger I, Boschert T, Jähne K, Green EW, Hülsmeyer I, Jünger S, Kessler T, Suwala A, Sahm F, Eisele P, Breckwoldt MO, Vajkoczy P, Grauer OM, Herrlinger U, Tonn JC, Denk M, Bendszus M, von Deimling A, Winkler F, Wick W, Platten M*, Sahm K*. A H3K27M-targeted vaccine in adults with diffuse midline glioma. Nat Med. 2023. 29(10):2586-2592.
DOI: 10.1038/s41591-023-02555-6
3. Grassl N*, Sahm K*, Süße H, Poschke I, Bunse L, Bunse T, Boschert T, Mildenberger I, Rupp AK, Ewinger MP, Lanz LM, Denk M, Tabatabai G, Ronellenfitsch MW, Herrlinger U, Glas M, Krex D, Vajkoczy P, Wick A, Harting I, Sahm F, von Deimling A, Bendszus M, Wick W, Platten M. INTERCEPT H3: A multicenter phase I peptide vaccine trial for the treatment of H3-mutated diffuse midline gliomas. Neurol Res Pract. 2023. 5(1):55.
DOI: 10.1186/s42466-023-00282-4
4. Kilian M, Sheinin R, Tan CL, Krämer C, Friedrich M, Kaminitz A, Sanghvi K, Lindner K, Chih YC, Cichon F, Richter B, Jung S, Jähne K, Ratliff M, Prins R, Etminan N, von Deimling A, Wick W, Madi A*, Bunse L*, Platten M*. MHC class II-restricted antigen presentation is required to prevent dysfunction of cytotoxic T cells by blood-borne myeloids in brain tumors. Cancer Cell. 2023. 41:1-17.
DOI: 10.1016/j.ccell.2022.12.007
5. Kober C, Roewe J*, Schmees N, Roese L, Roehn U, Bader B, Stoeckigt D, Prinz F, Gorjánácz M, Roider HG, Olesch C, Leder G, Irlbacher H, Lesche R, Lefranc J, Oezcan-Wahlbrink M, Sati Batra A, Elmadany N, Carretero R, Sahm K, Oezen I, Cichon F, Baumann D, Sadik A, Opitz C, Weinmann H, Hartung IV, Kreft B, Offringa R, Platten M*, Gutcher I* (2023). Targeting the aryl hydrocarbon receptor (AhR) with BAY 2416964: a selective small molecule inhibitor for cancer immunotherapy. J Immunother Cancer. 2023. 11(11):e007495.
DOI: 10.1136/jitc-2023-007495.
6. Bunse L, Rupp AK, Poschke I, Bunse T, Lindner K, Wick A, Blobner J, Misch M, Tabatabai G, Glas M, Schnell O, Gempt J, Denk M, Reifenberger G, Bendszus M, Wuchter P, Steinbach JP, Wick W, Platten M. AMPLIFY-NEOVAC: a randomized, 3-arm multicenter phase I trial to assess safety, tolerability and immunogenicity of IDH1-vac combined with an immune checkpoint inhibitor targeting programmed death-ligand 1 in isocitrate dehydrogenase 1 mutant gliomas. Neurol Res Pract. 2022. 23;4(1):20. DOI: 10.1186/s42466-022-00184-x
7. Platten M, Bunse L, Wick A, Bunse T, Le Cornet L, Harting I, Sahm F, Sanghvi K, Tan CL, Poschke I, Green E, Justesen S, Behrens GA, Breckwoldt MO, Freitag A, Rother LM, Schmitt A, Schnell O, Hense J, Misch M, Krex D, Stevanovic S, Tabatabai G, Steinbach JP, Bendszus M, von Deimling A, Schmitt M, Wick W. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature. 2021. 592:463-468.
DOI: 10.1038/s41586-021-03363-z
8. Friedrich M, Sankowski R, Bunse L, Kilian M, Green E, Ramallo Guevara C, Pusch S, Poschet G, Sanghvi K, Hahn M, Bunse T, Münch P, Gegner HM, Sonner JK, von Landenberg A, Cichon F, Aslan K, Trobisch T, Schirmer L, Abu-Sammour D, Kessler T, Ratliff M, Schrimpf D, Sahm F, Hopf C, Heiland DH, Schnell O, Beck J, Böttcher C, Fernandez-Zapata C, Priller J, Heiland S, Gutcher I, Quintana FJ, von Deimling A, Wick W, Prinz M*, Platten M*. Tryptophan metabolism drives dynamic immuno-suppressive myeloid states in IDH-mutant gliomas. Nat Cancer. 2021. 2:723-740.
DOI: 10.1038/s43018-021-00201-z
9. Aslan K, Turco V, Blobner J, Sonner JK, Liuzzi AR, Núñez NG, De Feo D, Kickingereder P, Fischer M, Green E, Sadik A, Friedrich M, Sanghvi K, Kilian M, Cichon F, Wolf L, Jähne K, von Landenberg A, Bunse L, Sahm F, Schrimpf D, Meyer J, Alexander A, Brugnara G, Röth R, Pfleiderer K, Niesler B, von Deimling A, Opitz CA, Breckwoldt MO, Heiland S, Bendszus M, Wick W, Becher B, Platten M. Heterogeneity of response to immune checkpoint blockade in hypermutated experimental gliomas. Nat Commun. 2020. 11:931.
DOI: 10.1038/s41467-020-14642-0
10. Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, Alansary D, Sonner JK, Green E, Deumelandt K, Kilian M, Neftel C, Uhlig S, Kessler T, von Landenberg A, Berghoff AS, Marsh K, Steadman M, Zhu D, Nicolay B, Wiestler B, Breckwoldt MO, Al-Ali R, Karcher-Bausch S, Bozza M, Oezen I, Kramer M, Meyer J, Habel A, Eisel J, Poschet G, Weller M, Preusser M, Nadji-Ohl M, Thon N, Burger MC, Harter PN, Ratliff M, Harbottle R, Benner A, Schrimpf D, Okun J, Herold-Mende C, Turcan S, Kaulfuss S, Hess‐Stumpp H, Bieback K, Cahill DP, Plate KH, Hänggi D, Dorsch M, Suvà ML, Niemeyer BA, von Deimling A, Wick W, Platten M. Suppression of antitumor T cell immunity by the oncometabolite R-2-hydroxyglutarate. Nat Med. 2018. 24:1192-1203.
DOI: 10.1038/s41591-018-0095-6
11. Bunse L, Schumacher T, Sahm F, Pusch S, Oezen I, Rauschenbach K, Gonzalez M, Solecki G, Osswald M, Capper D, Wiestler B, Winkler F, Herold-Mende C, von Deimling A, Wick W, Platten M. Proximity ligation assay evaluates IDH1R132H presentation in gliomas. J Clin Invest. 2015. 125:1-14.
DOI: 10.1172/JCI77780
12. Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, Menn O, Osswald M, Oezen I, Ott M, Keil M, Balß J, Rauschenbach K, Grabowska AK, Vogler I, Diekmann J, Trautwein N, Eichmüller SB, Okun J, Stevanović S, Riemer AB, Sahin U, Friese MA, Beckhove P, von Deimling A, Wick W, Platten M. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014. 512:324-327.
DOI: 10.1038/nature13387
13. Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous ligand of the human aryl hydrocarbon receptor promotes tumor formation. Nature 2011. 478:197-203.
DOI: 10.1038/nature10491
14. Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, Gupta R, Lee LY, Kidd BA, Robinson WH, Sobel RA, Selley ML, Steinman L. Treatment of autoimmune neuro-inflammation with a synthetic tryptophan metabolite. Science. 2005. 310:850-855.
DOI: 10.1126/ science.1117634
Context Column
EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood
Weller, M., van den Bent, M., … Platten, M., et al., Nat Rev Clin Oncol (2021).
https://doi.org/10.1038/s41571-020-00447-z