Sie befinden sich hier
Inhalt
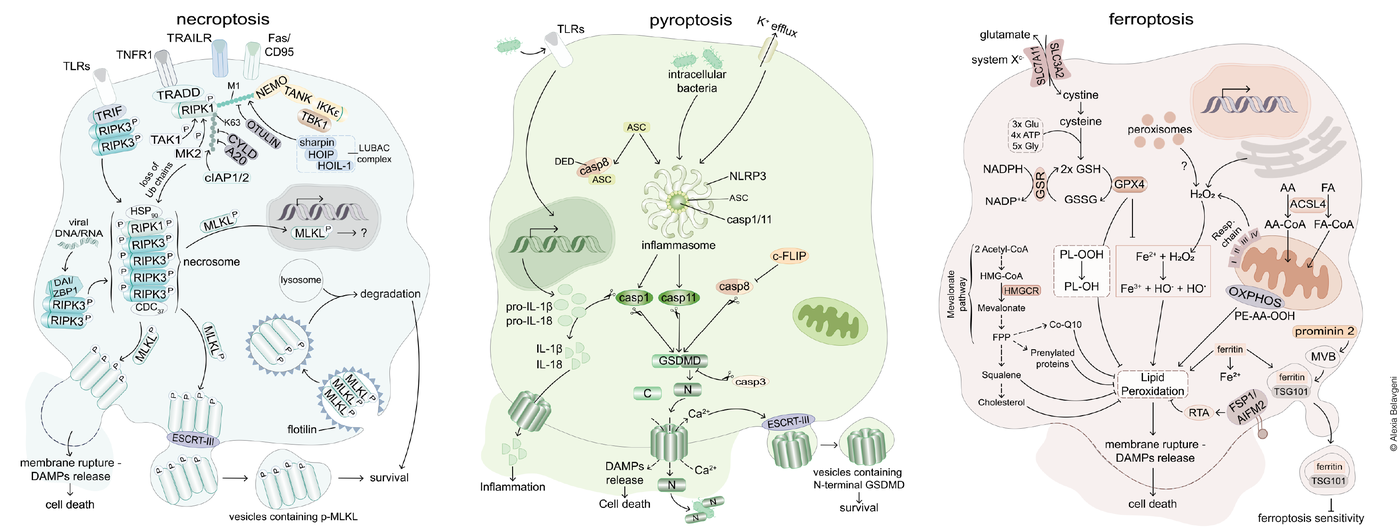
The signaling pathways of regulated necrosis. In general, two systems of regulated cell death may be best differentiated. The caspase-controlled system includes necroptosis and pyroptosis, have been studied in great detail. In contrast, the peroxidation-controlled system of ferroptotic cell death functions entirely independent of the caspase- controlled network. Importantly, both systems independently contribute to acute kidney injury. The necrotic signaling pathways are associated with the release of damage associated molecular patterns (DAMPs) which explain the immunogenicity of necrotic cell death pathways. Adapted from Tonnus et al., Nat Rev Endo 2021
Necroptosis
Necroptosis, a form of regulated necrosis, has been increasingly recognized for its role in acute kidney injury (AKI) among other clinical implications. This signaling pathway involves the receptor-interacting protein kinases 1 and 3 (RIPK1 and RIPK3) which can lead to a phosphorylation of mixed lineage kinase domain-like pseudokinase (MLKL). This causes an oligomerization of MLKL, leading to membrane blebbing, vesicle formation and ultimately loss of membrane integrity.
Necroptosis contributes to tissue damage and inflammation in AKI, with RIPK3 playing a critical role in promoting kidney tubular injury. This is supported by various experimental models, highlighting the potential for necroptosis inhibition as a therapeutic target. However, challenges remain due to the complexity of AKI and further research is needed to advance the understanding of the mechanism and to validate treatment options.
Pyroptosis
This form of regulated necrosis contributes to AKI and CKD, where excessive pyroptosis exacerbates inflammation and tissue damage. Pyroptosis is described as a form of regulated cell death following gasdermin D (GSDMD) cleavage, leading to membrane rupture and the release of pro-inflammatory cytokines such as IL-1β and IL-18. Mechanistically, pyroptosis is categorized into the caspase-1-mediated classical pyroptotic pathway and the caspase-4/5/11-mediated non-classical pyroptotic pathway and occurs downstream of inflammasome activation. A more detailed understanding of the mechanisms of this cell death pathway during kidney diseases can pave the way for novel therapeutic strategies in managing kidney diseases among others.
Ferroptosis
Ferroptosis, in contrast to necroptosis and pyroptosis, is characterized by iron-dependent lipid peroxidation and has emerged as a significant factor in the pathogenesis of myocardial infarction, stroke, acute kidney injury and others. Under normal conditions, ferroptosis is prevented by several molecular surveilling systems. The best characterized system is based on the function of the glutathione peroxidase 4 (GPX4), a glutathione (GSH) metabolizing selenoenzyme that converts oxidized lipids to respective non-toxic lipid alcohols. In addition, the GSH-independent coenzyme Q oxidoreductase FSP1 acts as another major regulator of ferroptosis by reducing CoQ10 to prevent lipid peroxidation, thus inhibiting ferroptosis. When these systems fail, membrane lipids are peroxidized, leading to the loss of plasma membrane integrity.
In organs sensitive to ferroptosis, a synchronized propagation of ferroptotic cell death has been observed (wave-of-death). These include heart tissue, brain and renal tubules, stating the relevance of this process and the medical need to study the mechanisms in detail.
Relevant publications