Sie befinden sich hier
Inhalt
Ribosomal proteins (RPs) in alcoholic liver disease (ALD) onset and progression
There is an increasing interest in elucidating the role of RPs in both normal physiological processes and in the pathogenesis of human diseases. Recent studies have shown that RPs may have extraribosomal functions that are involved in proliferation, differentiation, apoptosis, DNA repair, and other cellular processes. Dysfunction of RPs has been linked to development and progression of hematological, metabolic, and cardiovascular diseases, as well as cancer.
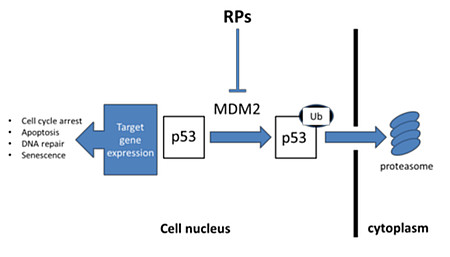
Nucleolar integrity is required to maintain ribosome biogenesis in the control of cell proliferation. Nucleolar stress, characterized by loss of nucleolar integrity, disturbs ribosome biogenesis and halts cell proliferation.1-2 Perturbation of ribosome biogenesis induces ribosomal stress, which triggers activation of the p53 signaling pathway through RPs–MDM2 interactions. Nucleolar stress mediates redistribution of nucleolar proteins to the nucleoplasm and alters their interactions with MDM2, which results in p53 activation.1, 3
We are interested in the role of ribosomal proteins and the RPs-MDM2-p53 axis in onset and development of ALD, a major health problem with rising prevalence across Europe. The development of new strategies to treat ALD is limited by the drawbacks of existing animal models.4 The National Institute on Alcohol Abuse and Alcoholism (NIAAA) has recently standardized an improved mouse model for the study of ALD.5
The experimental design leads to alcoholic fatty liver, steatohepatitis and fibrosis, making it the most adequate model available today to study ALD onset and progression. Moreover, this model is suitable to be used in combination with transgenic mice or diets to study the effects of alcohol in different settings.
Based on our previous findings, suggest that the cluster of RP genes are dose-dependently up-regulated and show a dysregulation of p53 downstream genes in rat livers challenged by long-term voluntary high alcohol consumption, we are about to establish several feeding protocols of the NIAAA model to further investigate these findings. Why liver cells respond with these changes, which are the mechanisms and how to translate these findings to the human situation are overarching aims of our research.
Publications
- Wang W, Nag S, Zhang X, Wang MH, Wang H, Zhou J, Zhang R. Ribosomal proteins and human diseases: pathogenesis, molecular mechanisms, and therapeutic implications. Med Res Rev. 2015 Mar; 35(2):225-85.
- Boulon S, Westman B J, Hutten S, Boisvert FM, Lamond AI. The nucleolus under stress. Mol Cell2010;40(2):216–227.
- Nag S, Qin JJ, Srivenugopal K, Wang MH, Zhang R. The MDM2-p53 pathway revisited. J BiomedRes 2013; 27(4):254–271
- Liu Y, Meyer C, Xu C, Weng H, Hellerbrand C, ten Dijke P, Dooley S. Animal models of chronic liver diseases. Am J Physiol Gastrointest Liver Physiol 2013;304:G449-G468
- Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 2013;8:627-637