Sie befinden sich hier
Inhalt
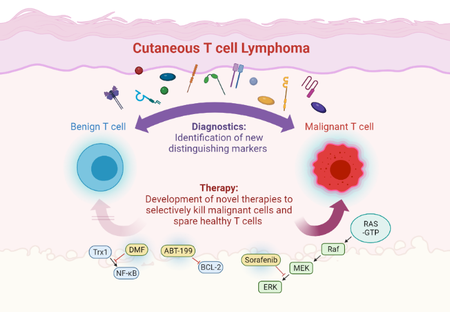
Cutaneous T cell lymphomas (CTCL) comprise a group of malignant lymphoproliferative diseases that primarily affect the skin, but can in high stages also spread to blood, lymph nodes or visceral organs. CTCL challenge both diagnostic and therapeutic management for several reasons: first, there are no unique markers to clearly differentiate the malignant T cell population from healthy T cells, so that diagnosis is often difficult and delayed. Second, there are no curative treatment options yet. Third, established treatments are often limited by their efficacy or tolerability.
So, there is an urgent need to establish novel diagnostic markers to improve and accelerate clear CTCL diagnosis as well as to develop new therapeutic options with higher efficacy and less side effects. The goal of our research is to solve both diagnostic and therapeutic challenges in CTCL in order to improve the disease course and quality of life for our patients.
To this end, we aim to understand the biology of the malignant T cells and therefore study cellular and molecular alterations in these cells. Upon their identification, we then check their quality as diagnostic markers as well as their potential as a therapeutic target. This approach proved very successful in recent years, as we were able to both establish new diagnostic CTCL markers and to implement novel targeted treatment options that we translate from preclinical experimental work into patient care by performing clinical studies.
Research highlights
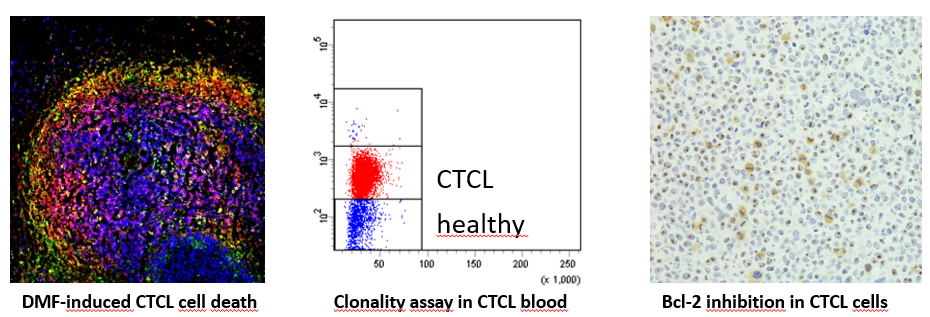
- Novel targeted therapies in CTCL: Therapy of CTCL is a great challenge due to short duration of response and limited tolerability of the available treatment options. Therefore, our research is focused on developing novel therapies that specifically target the distinct cell death resistance of CTCL cells and spare benign bystander T cells (Kiessling*, Nicolay* et al.: Oncotarget 2017). In this context, we identified dimethyl fumarate (DMF) as such a promising candidate in preclinical studies (Nicolay JP et al.: Blood 2016). In a translational approach, we also performed a clinical phase II multicenter study to confirm the effects also in a clinical context. In addition, we try to find novel targeted combination therapies to increase efficacy and tolerability. In this context, we found synergistic effects for the combination of DMF with Bcl-2 inhibition and extracorporeal photopheresis (Fröhlich et al.: Blood 2019).
- Novel diagnostic markers and therapeutic targets in CTCL: In order to improve and accelerate CTCL diagnosis we aim to develop novel diagnostic markers. CTCL cells differ from benign T cells by several alterated signaling pathways and aberrant surface molecule expression. Here, we study malignant T cells from the skin and the blood to characterize their marker profile (Ruggiero*, Nicolay* et al.: Nature Communications 2015; Nicolay et al.: JDDG 2016). By correlation with clonality analyses and cell death resistance we can identify the markers that describe the malignant population best and thus might serve as novel therapeutic targets.
- Evaluating quality of life and response to therapy in CTCL: In order to improve the current therapeutic management of our patients, we perform several clinical investigations that accompany therapy. In these non-interventional studies, we assess the course of quality of life for our patients as well as biomarkers and response/side effects during therapy. By correlating these factors, we aim to improve patient stratification. These newly started projects are supported by several funding organizations and industry.
- Clinical studies in CTCL research: In order to evaluate novel therapeutic strategies, we are study center for clinical CTCL therapy studies. As one of very few centers around Europe, we take part in all CTCL multicenter studies that currently test novel therapies in this disease group in Europe. In addition, as part of the EORTC board, we are involved in planning and designing novel studies on combination therapies that are about to start (EORTC 1820/MOGAT). In addition, we have initiated our own clinical therapy study to investigate DMF, an inhibitor of the NFκB pathway that is dysregulated in cutaneous T-cell lymphomas, in order to establish it as a clinically applicable therapeutic.
Kontakt
Prof. Dr. med. Jan P. Nicolay
Klinik für Dermatologie, Venerologie und Allergologie Universitätsmedizin Mannheim und Medizinische Fakultät Mannheim der Universität Heidelberg Haus 27; Ebene 4 Theodor-Kutzer-Ufer 1-3 68167 Mannheim
Telefon 0621/383-2280
Telefax 0621/383-3815
E-Mail jan.nicolay@umm.de